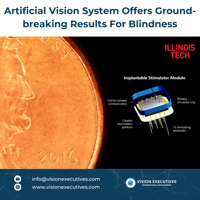
🔬 A New Frontier in Medical Science for #Eyecare 👁️ Artificial vision systems are pushing the...
𝐅𝐃𝐀 𝐀𝐩𝐩𝐫𝐨𝐯𝐞𝐬 𝐍𝐞𝐮𝐫𝐨𝐭𝐞𝐜𝐡’𝐬 𝐄𝐍𝐂𝐄𝐋𝐓𝐎 𝐂𝐞𝐥𝐥 𝐓𝐡𝐞𝐫𝐚𝐩𝐲 𝐟𝐨𝐫 𝐌𝐚𝐜𝐓𝐞𝐥
👏 The FDA has approved Neurotech Pharmaceuticals, Inc. Pharmaceuticals' ENCELTO (revakinagene taroretcel-lwey), the first treatment for macular telangiectasia type 2 (MacTel)—a progressive retinal disease causing vision loss.
👨⚕️ ENCELTO uses Encapsulated Cell Therapy (ECT), implanting genetically engineered cells that continuously release ciliary neurotrophic factor (CNTF) to slow retinal degeneration. Phase 3 trials showed significant preservation of macular photoreceptors over 24 months.
🌎 Neurotech’s CEO hailed the approval as a milestone, and experts believe ENCELTO will help patients maintain functional vision. The treatment is expected to launch in the U.S. by June 2025.
👏 The FDA has approved Neurotech Pharmaceuticals, Inc. Pharmaceuticals' ENCELTO (revakinagene taroretcel-lwey), the first treatment for macular telangiectasia type 2 (MacTel)—a progressive retinal disease causing vision loss.
👨⚕️ ENCELTO uses Encapsulated Cell Therapy (ECT), implanting genetically engineered cells that continuously release ciliary neurotrophic factor (CNTF) to slow retinal degeneration. Phase 3 trials showed significant preservation of macular photoreceptors over 24 months.
🌎 Neurotech’s CEO hailed the approval as a milestone, and experts believe ENCELTO will help patients maintain functional vision. The treatment is expected to launch in the U.S. by June 2025.