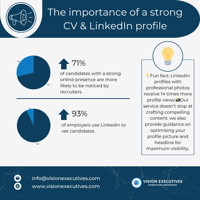
Your CV & LinkedIn profile are your digital handshake in the professional world🤝 In today’s...
𝐎𝐫𝐚𝐬𝐢𝐬 𝐏𝐡𝐚𝐫𝐦𝐚𝐜𝐞𝐮𝐭𝐢𝐜𝐚𝐥𝐬 𝐒𝐞𝐜𝐮𝐫𝐞𝐬 $78 𝐌𝐢𝐥𝐥𝐢𝐨𝐧 𝐭𝐨 𝐒𝐮𝐩𝐩𝐨𝐫𝐭 𝐔.𝐒. 𝐋𝐚𝐮𝐧𝐜𝐡 𝐨𝐟 𝐐𝐥𝐨𝐬𝐢 𝐟𝐨𝐫 #𝐏𝐫𝐞𝐬𝐛𝐲𝐨𝐩𝐢𝐚
💰 Nearly a year after receiving FDA approval, Orasis Pharmaceuticals has successfully raised $78 million in financing to back the U.S. commercial launch of its ground breaking product, Qlosi (pilocarpine hydrochloride ophthalmic solution) 0.4%. This preservative-free eye drop, originally developed under the name CSF-1, is specifically designed to treat presbyopia—a common age-related condition that affects near vision.
🌎 The new funding will be instrumental in bringing Qlosi to the market, supporting its launch and expanding its reach across the country. This development comes at a time when the pharmaceutical industry is focusing on innovative solutions to meet the growing needs of patients with presbyopia. With Qlosi’s approval, Orasis is positioning itself as a key player in this expanding market.