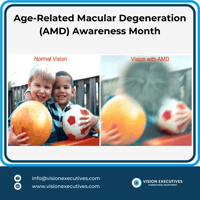
📢 February is Age-Related Macular Degeneration Awareness Month 👁️ Age-Related Macular...
👁 Genentech, a member of the Roche group, has received FDA approval to relaunch Susvimo (ranibizumab injection 100 mg/mL) for treating wet (or neovascular) age-related macular degeneration (AMD). This innovative treatment involves a refillable ocular implant, about the size of a grain of rice, which is placed under the upper eyelid. The implant continuously releases a specialised formulation of ranibizumab, providing a steady delivery of medication via the Port Delivery Platform. This method contrasts with other treatments that require multiple eye injections annually.
🙌 In October 2022, Genentech voluntarily recalled Susvimo due to a device issue detected during commercial supply testing. With the problem now resolved, Susvimo is ready to offer patients a more convenient and continuous treatment option for wet AMD.